Abstract
People living with type 1 Diabetes (T1D) and their families have poor perception of health related quality of life. Therapies for T1D are becoming better with time, but they still involve a lot of effort. Prevention of T1D, if successful, has potential to change lives of millions of families across the globe. Type 1 diabetes is an autoimmune disease with underlying genetic predisposition for autoimmunity against beta cell antigens upon exposure to an environmental trigger. Identifying underlying primary antigen responsible for initiating autoimmune cascade, avoiding environmental trigger and modifying immunity has all been used as strategies for preventing or delaying onset of type 1 diabetes. Primary prevention for type 1 diabetes is hindered by difficulty in identifying at-risk population and also due to lack of effective preventive strategy. Secondary prevention, in children with presence of autoimmunity, has recently received a boost with approval of Teplizumab, an immunity modifying drug by its Anti-CD3 action. Application of preventive strategies would also change based on country specific incidence, prevalence and availability of health resources. In current review, an update on preventive strategies for type 1 diabetes is being discussed as well as their applicability in Indian context.
Keywords: Indian perspective, prevention, teplizumab, type 1 diabetes
INTRODUCTION
Healthcare focus in India is gradually shifting from acute infectious communicable diseases to chronic non-communicable diseases.[1] Management of chronic diseases entails long-term follow-up and control of disease rather than a complete cure. Thus, prevention strategies are an integral part of managing chronic noncommunicable diseases. For successful implementation of a preventive strategy for a chronic disease at the public health level requires the fulfillment of the following criteria:
Disease should be of sufficient health concern to an individual and public
At-risk populations should be identifiable easily by available diagnostic measures
Prevention strategy should be effective enough to delay the onset or prevent the disease altogether.
Prevention strategy should be cost-effective to be implemented at the larger population level
India has the highest number of people, <20 years of age, living with Type 1 diabetes (T1D) among countries worldwide.[2] This is despite the relatively low incidence of T1D in India as compared to Scandinavian countries.[3] Type 1 diabetes incidence peaks at 5-9 years of age and then it requires lifelong intense treatment and monitoring. People living with T1D in India have a significantly shorter span of life than the general population.[3,4] People living with T1D and their families have a poor quality of life and an increased incidence of depression.[3,5-8] Glycemic control in people living with T1D remains suboptimal even in developed countries with mean HbA1c staying >8% and less than 30% affected people achieving HbA1c <7%.[9] Therapies of T1D are becoming better and more efficacious with time, it still involves a lot of effort and expenditure (out of pocket in the case of India).[10] These arguments make T1D an apt condition to be considered for the implementation of preventive strategies. Delaying the onset of T1DM by even a few years would be expected to decrease not only the disease burden but also the risk of long-term complications. In this narrative review, we discuss the current status of preventive strategies in the field of T1D and their applicability in the Indian context.
DEFINITION OF PREVENTION LEVELS IN TYPE 1 DIABETES
In general, prevention strategies are defined as primordial, primary, secondary, and tertiary prevention. At-risk people for T1D in the general population are difficult to identify as known risk factors for T1D are not well defined currently. Therefore, primordial prevention is not relevant to T1D as it involves modifying risk factors in the general population. The pathophysiology of T1D has been divided into 3 stages to facilitate the implementation of prevention strategies appropriately [Table 1].[11]
Table 1.
Stages of type 1 diabetes
| Pre-stage 1 | Stage 1 | Stage 2 | Stage 3 | |
|---|---|---|---|---|
| Risk Factors | Present | ± | ± | ± |
| Beta-cell autoimmunity | - | Present | Present | Present |
| Glycemic Status | - | - | Dysglycemia | Hyperglycemia |
| Symptoms | - | - | - | Present |
Known risk factors, at present, include family history, number of first-degree relatives affected, age of onset in a family member (age of onset <7 years confers greater risk), genetic predisposition with the presence of predisposing HLA type and also non-HLA gene loci like INS, PTPN22, IL2RA among others.[12] More than 50 non-HLA genetic loci have been identified to confer risk for T1D.[13,14] Beta-cell immunity is identified by the presence of various autoantibodies against islet called antigens and includes anti-insulin antibodies, anti-insulinoma associated-2 antibodies (IA-2), anti-GAD 65 antibodies, and anti-ZnT8 antibodies. Dysglycemia in stage 2 is defined as glycemic levels in the pre-diabetes range on an oral glucose tolerance test. Based on these stages prevention levels in T1D can be defined in Table 2.
Table 2.
Definition of prevention levels in terms of type 1 diabetes
| Level | Definition in general | Definition in terms of T1D |
|---|---|---|
| Primary | A disorder is actually prevented from developing | Individuals at high risk of developing T1D and aimed at preventing the autoimmunity against islet autoantigens |
| Secondary | Disease is detected and treated early, often before symptoms are present | Individuals with multiple islet autoantibodies with the aim of halting autoimmunity processes and possibly avoid the clinical onset of diabetes |
| Tertiary | An existing, usually chronic disease is managed to prevent complications or further damage | Stage 3 clinical type 1 diabetes (i.e., classic symptomatic type 1 diabetes requiring insulin therapy), mostly recent onset, but some in established disease |
For the purpose of implementing prevention strategies, clarity should be sought on at-risk identifiable populations and proposed prevention strategies at that level of prevention.
PRIMARY PREVENTION
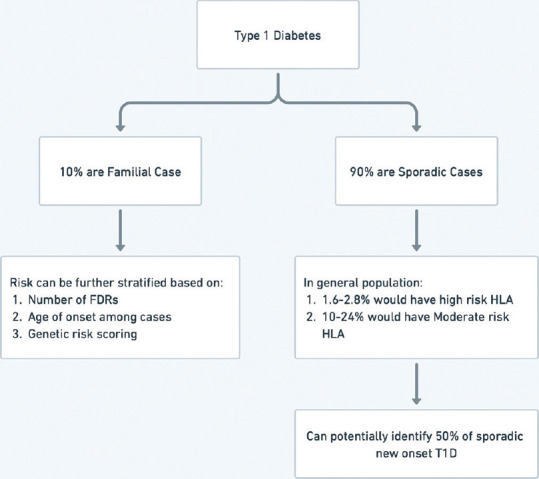
The population at risk for T1D practically includes the entire childhood population as risk factors for T1D are not completely elucidated. Among new onset T1D, 10-15% of cases are familial in nature.[11] In other words, if all first-degree relatives (FDRs) of T1D cases are followed, 10-15% of new cases of T1D can potentially be identified.[11] Relative risk varies according to association with the index case. In data from the United States of America, the risk of T1D in a newborn without any affected FDR is 0.4-1% and in a newborn with one affected FDR risk is ~5%.[11] Risk can be as high as 20-25% in people with multiple affected FDRs and 30-70% in monozygotic twins.[11] As identifying these people is relatively simple, most of the preventive strategies are tested and proven in this group.
Genetic risk scores have been devised based on the HLA genotype and non-HLA gene loci. Elucidating this risk can identify up to 50% of new cases of T1D. Major HLA alleles, from Western countries, predisposing for T1D include HLA B8 (OR 3.20), HLA B15 (OR 3.69), HLA-DR3 (OR 3.54), and HLA-DR4 (OR 6.81).[15] Protective alleles include HLA-DR2 (OR 0.21), HLA-DR5 (OR 0.30), and HLA-DR7 (OR 0.24).[15] In a study from North India, HLA-DRB1*03, DQA1*05, and DQB1*02 showed significant positive associations with T1D, whereas HLA-DRB1*15, DQA1*01, and DQB1*06 were negatively associated with T1D.[16] There was also an increased frequency of HLA-DRB1*04 among people living with T1D in a north Indian population.[16] However, this association of DR4 and T1D is not independent of DR3 as most of the patients carrying DRB1*04 also carried DRB1*03.[16] From HLA Class 1 gene, a positive association was reported between HLA-A*02, A*26, B*08, and B*50, and T1D in subjects from north India.[16] However, testing for these alleles is costly at present and may not be implementable at the public health level, especially in countries like India with low incidence rates. Almost a quarter of the general population would have a high or moderate risk HLA allele.[17] So, to predict one case of T1D more than 2000 children need to be followed up. This should also take into account the psychological stress and anxiety in children who are eventually not going to be suffering from T1D [Figure 1].
Figure 1.
Identifying population-at-risk for primary prevention of type 1 diabetes
Another factor that needs to be considered at the primary prevention level is that T1D develops in a genetically predisposed child only when a second hit from some environmental factor induces autoimmunity against beta cells.[12] So, while genetically predisposed persons may still be identified based on genetic testing, the occurrence of T1D in this population may still be random and non-linear.
Primary prevention strategies for T1D need to be essentially interventions that have a very low potential of inducing harm as the base population is very high and a large majority of them are not going to develop the disease at all. These strategies essentially include avoidance of exposure to suspected environmental factors in genetically predisposed populations.[12] However, elucidation and defining these environmental factors is still a work in progress. Attempts have also been made to devise therapies that modulate immunity in general or in relation to antigens specific to pancreatic islets by the principle of bystander suppression.[18]
Environmental factors worth mentioning include exposure to cow’s milk and gluten. In a meta-analysis involving 13 case-control studies, diagnosis of type 1 diabetes was associated with early weaning at <3 months of age (overall OR 1.43; 95% CI 1.15-1.77) and early exposure to cow’s milk at <4 months of age (overall OR 1.63; 95% CI 1.22-2.17).[19] Subsequent prospective studies have found some association between exposure to cow’s milk and the development of autoimmunity.[20] But this association is limited to the development of one type 1 diabetes-specific autoantibody. After 7 years of follow-up, when the development of 2 or more antibodies has been considered as an end point, no difference has been noted.[20] Using bovine insulin-free formulas have also resulted in lower development of one or more type 1 diabetes specific autoantibody.[21] As the development of one autoantibody does not predict the development of type diabetes robustly, avoiding cow’s milk or using bovine insulin-free formulas in the large population does not make a sound scientific argument. Similarly, late exposure to gluten (at 12 months of age vs 6 months of age) revealed no difference in the development of T1D-specific autoimmunity.[22]
Therapies involving anti-inflammatory action or immunity-modulating action in general, like the use of docosahexaenoic acid, vitamin D, and high-dose nicotinamide, have been tried with unsuccessful results.[12,23] However, antigenic-specific therapies to induce immune desensitization using the principle of bystander suppression continue to pique the interest of scientists.
Bystander suppression involves exposure to an antigen similar to a target antigen (against which autoimmunity is to be avoided). This exposure is believed to induce the development of helper T cells which induce immune tolerance to target antigens by immune spillover. This strategy has not only been tested as a primary prevention strategy but also at the secondary prevention level. Type 1 diabetes is hypothesized to develop by epitope spreading, where autoimmunity to one particular antigen leads to beta cell damage with the release of further beta cell antigens. This leads to a cascade of autoimmunity to a number of beta-cell antigens. However, the primary antigen responsible for the start of this cascade is still elusive. Proinsulin has been proposed to be initiating antigen but the final word is still to be spelled.[24] But this has led to a number of trials involving the administration of insulin to at-risk children via oral, parenteral, or nasal route.[12] In a dose-finding study, an immune response to insulin was measured as serum IgG and saliva IgA binding to insulin, and CD4+ T-cell proliferative responses to insulin after administration of oral insulin.[25] Oral administration of 67.5 mg on a daily basis resulted in the development of an immune response.[25] However, most of the clinical studies are being done at the secondary prevention level and would be discussed in subsequent sections.
To summarize the current status of primary prevention:
Only a small fraction of new-onset T1D cases can be identified by clinical history. Further at-risk cases can be identified using genetic screening, but a large number of children would need to be tested. Psychological stress placed on children and their families who would be screened positive but would not develop T1D also needs to be considered.
There has been very limited success, if at all, in identifying therapies that can be implemented at the population level to primarily prevent T1D.
There is no clinical guidance by any scientific group to implement primary prevention strategies currently.
SECONDARY PREVENTION
Secondary prevention for T1D involves preventing or delaying the onset of clinical disease in people who are in stage 1 and stage 2 of T1D development. This spectrum includes people who already have 2 or more T1D-specific autoantibodies to people who not only have autoantibodies but also have raised blood glucose values not fulfilling the criteria for diagnosis of diabetes.[11] It is to be noted here that the development of one autoantibody has not been considered enough to qualify for stage 1 of T1D development. In a landmark study with 15 years follow-up, the development of multiple antibodies (2 or more) was associated with the development of T1D in 69.7% of people, while 14.5% and 0.4% of children developed T1D with one or no antibodies, respectively.[26]
Autoantibodies usually develop after 6 months of age and peak at 9-24 months.[27] The development of one antibody at less than 5 years of age and the presence of genetic predisposition enhances the chances of developing a second antibody. However, seroconversion from the first antibody to the second antibody is rare after 4 years of follow-up.[11] After the development of 2 or more autoantibodies, there may not be any metabolic decline for years. Dysglycemia may be detected almost 1.5 years prior to clinical onset (stage 3) of T1D.[11] Beta cell function/C peptide levels decline accelerates 6 months prior to clinical onset with a rapid decline in the last 3 months.[11]
The presence of autoantibodies and dysglycemia objectively defines the population eligible for secondary prevention. This population would also contribute to the majority of new cases of T1D in any community. However, as children are still asymptomatic at this stage, the question regarding whom to screen for autoantibodies and dysglycemia still remains unanswered. Countries with high prevalence, low absolute children population, and sufficient public health expenditure are trying to devise strategies to implement these screenings at the whole population level.[28]
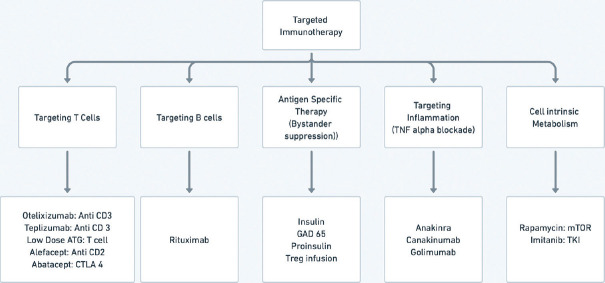
Strategies used for secondary prevention essentially involve immunomodulation either by bystander suppression, anti-inflammatory action at the beta cell level, or by modifying the action of either T cell or B cell. An overview of these therapies is shown in Figure 2.
Figure 2.
Overview of immunity modifying therapies for prevention of type 1 diabetes
Proinsulin, insulin (oral, parenteral, and nasal route), and GAD 65 have all been used to induce bystander suppression of autoimmunity towards islet cells. As a part of the effort towards secondary prevention, these agents have been administered to FDRs of T1D cases, who the have presence of autoantibodies with or without further risk stratification by either genetic testing or the presence of dysglycemia. Diabetes prevention trial (DPT) - 1 with parenteral insulin (subcutaneous daily and IV infusion for 4 days in a year) did not delay/prevent the onset of T1D.[29] In the oral DPT, there was a decrease in the incidence of T1D in a subset of people with a high titer of insulin autoantibody when 7.5 mg insulin per day was given as an intervention over 4.3 years. 10.4% of people in the placebo group developed T1D as compared to 6.2% in the oral insulin group (HR = 0.566, P = 0.015).[30] In TrialNet Oral Insulin Study, follow-up of over 8 years revealed the benefit of oral insulin in a subset of the study group. In a subset of children (n = 55) with 2 or more autoantibodies and with declining beta cell function (measured by first-phase insulin response and C-peptide levels), oral insulin 7.5 mg/day delayed onset of T1D by 31 months.[31] However, oral insulin was not effective in a larger subset an entire cohort.[31] Other studies with oral insulins, nasal insulin, or GAD 65 have also not shown promising outcomes.
The pre-point study, where investigators tried to find a dose of oral insulin to trigger immune responses initiated introspection on the adequacy of intervention in oral insulin trials.[25] In the Pre-point study, oral insulin doses from 2.5 mg to 67.5 mg were given to 9 children, and their immune responses were measured. Children participating in this study were relatives of people with T1D and had susceptible genotypes but had no islet cell autoimmunity. Immune response to insulin was measured as serum IgG and saliva IgA binding to insulin, and CD4+ T-cell proliferative responses to insulin after administration of oral insulin. Children given up to 7.5 mg/day of oral insulin had an immune response similar to the placebo group (16.7% vs 20% respectively). Children given 22.5 mg/day had a response (33.3%) marginally higher than placebo but the difference was not statistically significant. On the other hand, 83.3% of children receiving 67.5 mg showed an immune response. Thus, less than expected results from antigen-specific therapies involving insulin therapy can be due to lower doses of insulin used. The trialnet orał insulin study group has already initiated studies with higher doses of oral insulin.
Another factor that may be responsible for the suboptimal outcomes in these studies may be the age of the participant at the time of intervention. The mean age participants in DPT 1 oral (10.25 years), DIPP Nasal insulin (2.7 years in birth cohort and 7 years in sibling cohort), and Trialnet oral insulin study (8.2 years) is much higher than the usual onset of autoimmunity in these children (6 -24 months).[30-32] As these interventions are applied for secondary prevention, by definition, at the time of intervention autoimmunity has already set in. It may be, hypothetically, more difficult to modify an already pre-existing immune response. So, antigen-specific therapy may be more useful if applied at younger ages and may be more effective at the primary prevention level.
Promising results have been reported with therapies modifying T-cell immune responses. The underlying hypothesis involves a down-regulation of either effector T cells or up-regulation of T helper cells. T cell antigens that have been targeted for this purpose include CD3, CD2, and CTLA4. As immunotherapies are targeted towards suppression or modifying disordered immune responses, these are apt for application for secondary prevention or very early stage 3 T1D. A summary of immunotherapies targeted at modifying immune response for T1D is delineated in Table 3.[33]
Table 3.
Summary of immunotherapies for type 1 diabetes
| Targeted Antigen | Agent | Proposed Mechanism | Current Status |
|---|---|---|---|
| CD3, T cell | Teplizumab[34] | Reduced T cell activation and up regulation of regulatory T cells in long run | Showed delay in onset of T1D by 24 months. Approved by FDA for secondary prevention |
| CD3, T cell | Otelixizumab[35] | Reduced T cell activation and up regulation of regulatory T cells in long run | Also delayed onset of T1D but effective dose seem to have significant incidence of EBV activation. Dose finding studies are on-going. |
| CD2, T cell | Alefacept[36] | Increased ratio of T regulatory cells to T effector cells | Immunological response and delayed decrease in C-peptide in clinical studies. Lower dose of insulin in alefacept group but no difference in HbA1c. Needs further studies. |
| CTLA 4, T cell | Abatacept[37] | Preventing T cell activation by blocking CD80 and CD86 on antigen-presenting cells. | Maintained higher C peptide during ongoing therapy. After discontinuation, decline in C peptide delayed by 9.6 months but parallel to placebo group. Further studies on-going. |
| CD 20, B cell | Rituximab[38] | Disruption of B cell function | Not effective alone. Ongoing studies in combination with abatacept. |
| TNF alpha | Golimumab[39,40] | Targets inflammation | Phase 2 study showed less need of insulin. Further results are awaited. |
Other therapies that have been found to be either ineffective or are under further study include therapies targeted toward Interleukin 1, Interleukin 2, anti-thymocyte globulin, mTOR pathway, and alpha 1 antitrypsin among others.[33] Teplizumab needs a special mention as this is the only FDA-approved therapy for the secondary prevention of T1D.
TEPLIZUMAB
The history of Teplizumab development can be traced back to the mid-1970 which saw the advent of B-cell hybridoma technology. This pushed the approach of therapeutics development towards monoclonal antibody (mAb) immunotherapy. The most successful clinical immunotherapy for T1D has been anti-CD3mAb.[41]
OKT3 is a murine monoclonal antibody of the immunoglobulin IgG2a isotype. It was developed in 1979 and received FDA approval as the first human mAb immunotherapy for the prevention of transplant rejection in 1986.[41] OKT3 worked by blocking the generation and function of cytotoxic T cells with the selective removal of CD3. However, its clinical implementation was restricted due to the “flu-like” side effects. The side effects were found to be the result of increased cytokine release from T-cells brought by the TCR (T cell receptor)/CD3 complex, induced by OKT3. Further, OKT3, being a mouse monoclonal antibody, results in the development of a human anti-mouse antibody (HAMA).[42] To help eliminate its fallibility, OKT3 was then humanized by inducing punctual mutations of the Fc thereby preventing binding to FcRs. This genetic engineering stripped off the mitogenic properties of OKT3 and resulted in the genesis of huCD3ε-directed mAb hOKT3γ1 Ala-Ala, also known as Teplizumab.
Teplizumab works by binding to CD3 of the autoreactive T cells (mediating the death of β-cells). Unlike OKT3 which causes the depletion of the autoreactive T cells, Teplizumab causes these cells to migrate to the gut wall and become exhausted.[43]
Interestingly, initial studies involving Teplizumab were done in new-onset T1D. In 2002, a U.S.-based phase I/II clinical trial was conducted with Teplizumab in patients with anti-islet autoantibodies positivity and recent (within the first 6 weeks of diagnosis) onset of T1D. It was noted that the insulin production was the same or better 1 year post-treatment in 9 out of 12 teplizumab recipients versus only 2 of the 12 placebo recipients.[44] Further, the glycosylated hemoglobin levels were found to be lower in the patients who had received teplizumab. The Autoimmunity-Blocking Antibody for Tolerance (AbATE) trial was set up to evaluate Teplizumab in individuals with new-onset T1D having an average follow-up duration of 8 years. Key messages coming from the AbATE trial were that 2 courses of teplizumab reduced the decline in C-peptide 2 years after the onset of the disease.[45] After 7 years of follow-up, the C-peptide responses to a mixed-meal tolerance test were found to be similar overall in the drug vs. control group of participants. However, they were significantly improved with less loss of C-peptide in drug-treated responders identified at 1 year.[46]
Finally, Teplizumab was shown to delay the onset of T1D in 76 patients with stage 2 type 1 diabetes by 24 months. The annualized rates of diagnosis of diabetes were 14.9% per year in the teplizumab group and 35.9% per year in the placebo group.[34] Based on this information, FDA USA approved Teplizumab for children who are 8 years or older and have stage 2 type 1 diabetes (with two or more diabetes-related autoantibodies and dysglycemia on OGTT but no symptoms).[47] A table summarizing basic information for the use of Teplizumab has been provided in Table 4.[48]
Table 4.
Prescribing information for Teplizumab49
| Indication: Teplizumab is a CD3-directed antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D. |
| Investigation before use: It is advisable to obtain a complete blood count and liver enzymes tests, prior to the initiation of teplizumab. |
| Premedicate with: |
| 1) Nonsteroidal anti-inflammatory drug (NSAID) or acetaminophen, |
| 2) An antihistamine, and/or |
| 3) An antiemetic before each teplizumab dose for at least the first 5 days of the 14-day treatment course. |
| Warnings And Precautions: |
| • Use of teplizumab is not recommended in patients with |
| 1) Lymphocyte count less than 1,000 lymphocytes/mcL |
| 2) Hemoglobin less than 10 g/dL |
| 3) Platelet count less than 150,000 platelets/mcL |
| 4) Absolute neutrophil count less than 1,500 neutrophils/mcL |
| 5) Elevated ALT or AST more than 2 times the upper limit of normal (ULN) or bilirubin more than 1.5 times ULN |
| 6) Laboratory or clinical evidence of acute infection with Epstein-Barr virus (EBV) or cytomegalovirus (CMV) |
| 7) Any active serious infection or chronic active infection (other than localized skin infections) |
| • Administer all age-appropriate vaccinations prior to starting teplizumab |
| 1) Administer live-attenuated (live) vaccines at least 8 weeks prior to treatment. |
| 2) Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. |
| Recommended Dosage and Administration Intravenous infusion administration of teplizumab (over a minimum of 30 minutes), using a body surface area-based dosing, once daily for 14 consecutive days as follows: |
| • Day 1: 65 mcg/m2 |
| • Day 2: 125 mcg/m2 |
| • Day 3: 250 mcg/m2 |
| • Day 4: 500 mcg/m2 |
| • Days 5 through 14: 1,030 mcg/m2 |
| Do not administer two doses on the same day. |
| Recommendations Regarding Missed Dose (s): Resume dosing by administering all remaining doses on consecutive days to complete the 14-day treatment course if a planned teplizumab infusion is missed. |
| Adverse Reactions: Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia and headache, cytokine release syndrome and serious infections. |
The use of Teplizumab in combination with other forms of cell replacement therapies is advocated for the possible prevention of T1D.
Herold et al.[34] observed that those individuals who had lower than median C peptide responses had favorable response to Teplizumab. Indirectly indicating that those individuals who have active autoimmunity have a favorable response to it. The same group also observed that those individuals who were HLA-DR3 negative, DR4 positive, and anti-ZnT8 antibody negative were more likely to respond to Teplizumab. It should be noted that Indians have different HLA phenotypes in T1DM and teplizumab needs to be tested considering this scenario.
It should be noted that just one infusion was studied by Herold KC et al.[34] Whether further infusions cause an impact to last longer needs to be examined. A phase 3 study called PROTECT, which involves patients with recently diagnosed clinical T1DM receiving two infusions spaced six months apart, is underway.[49] It investigated relatives of T1DM patients; it has to be determined if the results apply to those with a greater genetic risk of T1DM but no family history.
Teplizumab has provided the right kind of morale booster to the field of T1D prevention which had seen dismal results so far. Teplizumab delays the onset of T1DM by months-years and does not fully prevent it. Although this by itself is a significant milestone with the potential to reduce the burden of patients and caregivers, the future, we hope, should hold a promise for the total prevention of T1DM.
To summarize the current status of secondary prevention:
Population suitable for secondary prevention can be clearly defined by the presence of autoantibodies with or without dysglycemia. However, the question of how to identify this population without subjecting the entire children population to screening for these antibodies still needs to be answered.
Success and approval of Teplizumab have rekindled the hope of better therapies for secondary prevention of T1D.
TERTIARY PREVENTION
Immunotherapies being used for secondary prevention are also tried in early-onset T1D with the aim to induce remission. As in secondary prevention, these therapies have shown some preservation of beta cell function but have failed to induce any meaningful remission. A brief mention of BCG vaccination needs to be made in tertiary prevention. BCG vaccine has been proposed to shift glucose metabolism from overactive oxidative phosphorylation to aerobic glycolysis.[50] Observation retrospective data seem to suggest that childhood BCG vaccine may be beneficial for preventing/delaying the onset of T1D.[51] There are no intervention studies evaluating the BCG vaccine as primary preventive therapy. But, intervention studies have consistently shown no benefit of using BCG as a tertiary preventive therapy.[52-54] Immunosuppressive therapies with cyclosporine and anti-thymocyte globulin have been shown to induce transient remission but the continued need for administration and significant side effects preclude their widespread use.[55,56]
Although there were initial studies that showed favorable response[44] to Teplizumab for tertiary prevention, larger studies done in the same scenario found a not so favorable response.[57] Further studies are needed in this area.
Efforts for islet cell transplantation are also underway to improve the ease of therapy and quality of life of people with T1D. However, the de novo generation of beta cells at the commercial level is still a little ahead in the future. A recent breakthrough in the de novo development of glucose-sensing beta cells has raised hopes of a successful and viable islet cell transplantation.[58]
Further advancements in T1D therapies include artificial pancreas, machine learning algorithms for insulin dose determination, carbohydrate counting applications, and better basal and prandial insulins among others.[59,60] However, a detailed discussion of these modalities is beyond the scope of this review.
INDIAN PERSPECTIVE
India has the largest number of people living with T1D in the entire world. This is despite the relatively low incidence of T1D in India. However, care for T1D in India has not been able to match with Western standards. A comparison of T1D numbers and care parameters of India with a few developed countries is portrayed in Table 5.[2,3]
Table 5.
A comparison of T1D numbers and care parameters across countries
| Germany | Finland | USA | India | |
|---|---|---|---|---|
| Prevalent cases of T1D (0-19 years) | 35.1K | 5.4K | 157.9K | 229.4K |
| New cases of Type 1 Diabetes (0-19 years) | 3600 (3 in 10000) | 600 (7 in 10000) | 18,200 (2.6 in 10000) | 24000 (0.8 in 10000) |
| Population for Primary prevention | 1.07 cr (<15 year) | 8.6 lakh (<15 year) | 7 cr (<18 year) | 30 Cr (<15 year) |
| Life expectancy for a 10 year old child with T1D | >70 years | >70 years | 55-69 years | 25-39 years |
| Total health spending per capita in PPP international dollars | 7032 | 4897 | 11702 | 191 |
The life expectancy of people living with T1D is markedly lower than the general population and also lower than people living with T1D in other countries. Given the large population of children in India, the ability to screen them for genetic susceptibility or the presence of autoantibodies would require massive resources. However, given low public health resources that does not seem a feasible approach currently. The cost of Teplizumab is prohibitive even in developed countries. A cost-benefit analysis done by Mital et al.,[61] suggested that the current cost of 1,93,900 US dollars can only be justified when used in people with the highest likelihood of response viz. individuals without HLA-DR3, with HLA-DR4 alleles and negative ZnT8 antibody status. On conversion to INR, the amount translates into ₹15869987.88 as of the date (15th April 2023). This implies it would be out of reach of many Indians even if they have the indications for the teplizumab. Therefore, in India, we need to dedicate current resources to improving the quality and longevity of life of people with T1D.
There is a lack of dedicated education resources geared towards T1D and access to insulin also seems to be an issue for a large population. We also need to work on solutions that would work for Indian conditions in a cost-effective manner. For example, while insulin pump technology is improving each day, cost constraints in India may limit its use. Enhancing access to care essentials like insulin, glucometer, and glucometer strips, and technology-based solutions for delivering diabetes education, allowing easy carbohydrate counting, and insulin dose determination for subcutaneous insulin administration may be priority areas for T1D care policies in India.[59]
CONCLUSION
Recent success in preventive therapies for type 1 diabetes has rekindled the hope of diabetes-free life in susceptible individuals. Teplizumab has initiated the cascade (hopefully) of successful immunotherapies for secondary and tertiary prevention; identifying eligible people is still a problem to be solved though. However, there is still a long way to go in designing ideal prevention therapies for T1D. Ongoing primary and secondary prevention therapies may be useful in countries with high incidence rates with low populations and adequate health resources like Finland and Germany. Although improving the quality and longevity of life of people living with T1D is a priority in India, there is a need to explore novel ways to prevent T1D.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dandona L, Dandona R, Kumar GA, Shukla DK, Paul VK, Balakrishnan K, et al. Nations within a nation: Variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017;390:2437–2460. doi: 10.1016/S0140-6736(17)32804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Type 1 diabetes estimates in children and adults. [[Lastaccessed on 2023 Feb 20]]. Available from: https://diabetesatlas.org/idfawp/resource-files/2022/12/IDF-T1D-Index-Report.pdf .
- 3.International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021. Available from: https://www.diabetesatlas.org . [Google Scholar]
- 4.Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhavani N, Prince S, Menon AS, Abraham N, Pavithran PV, Menon UV, et al. Health related quality of life in pediatric onset Type 1 diabetes mellitus in Kerala, India. Pediatr Diabetes. 2021;22:369–73. doi: 10.1111/pedi.13151. [DOI] [PubMed] [Google Scholar]
- 6.Kalyva E, Malakonaki E, Eiser C, Mamoulakis D. Health-related quality of life (HRQoL) of children with type 1 diabetes mellitus (T1DM): Self and parental perceptions. Pediatr Diabetes. 2011;12:34–40. doi: 10.1111/j.1399-5448.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 7.Faulkner MS, Clark FS. Quality of life for parents of children and adolescents with type 1 diabetes. Diabetes Educ. 1998;24:721–7. doi: 10.1177/014572179802400607. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen HB, Ovesen LL, Mortensen LH, Lau CJ, Joensen LE. Type 1 diabetes, quality of life, occupational status and education level-A comparative population-based study. Diabetes Res Clin Pract. 2016;121:62–8. doi: 10.1016/j.diabres.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U. S.: Updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971–8. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 10.Singla R, Bindra J, Singla A, Gupta Y, Kalra S. Drug prescription patterns and cost analysis of diabetes therapy in India: Audit of an endocrine practice. Indian J Endocrinol Metab. 2019;23:40–5. doi: 10.4103/ijem.IJEM_646_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: A scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–74. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skyler JS, Krischer JP, Becker DJ, Rewers M. Prevention of type 1 diabetes. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, et al., editors. Diabetes in America. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018. [PubMed] [Google Scholar]
- 13.Sharp SA, Rich SS, Wood AR, Jones SE, Beaumont RN, Harrison JW, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care. 2019;42:200–7. doi: 10.2337/dc18-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redondo MJ, Gignoux CR, Dabelea D, Hagopian WA, Onengut-Gumuscu S, Oram RA, et al. Type 1 diabetes in diverse ancestries and the use of genetic risk scores. Lancet Diabetes Endocrinol. 2022;10:597–608. doi: 10.1016/S2213-8587(22)00159-0. doi:10.1016/S2213-858700159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rich SS, Erlich H, Concannon P. Genetics of type 1 diabetes. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, et al., editors. Diabetes in America. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018. [PubMed] [Google Scholar]
- 16.Kumar N, Mehra NK, Kanga U, Kaur G, Tandon N, Chuzho N, et al. Diverse human leukocyte antigen association of type 1 diabetes in north India. J Diabetes. 2019;11:719–28. doi: 10.1111/1753-0407.12898. [DOI] [PubMed] [Google Scholar]
- 17.Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, et al. Newborn screening for HLA markers associated with IDDM: Diabetes autoimmunity study in the young (DAISY) Diabetologia. 1996;39:807–12. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 18.Roep BO, Wheeler DCS, Peakman M. Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 2019;7:65–74. doi: 10.1016/S2213-8587(18)30109-8. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC. Cow's milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care. 1994;17:13–9. doi: 10.2337/diacare.17.1.13. [DOI] [PubMed] [Google Scholar]
- 20.Knip M, Virtanen SM, Seppä K, Ilonen J, Savilahti E, Vaarala O, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363:1900–8. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaarala O, Ilonen J, Ruohtula T, Pesola J, Virtanen SM, Härkönen T, et al. Removal of bovine insulin from cow's milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch Pediatr Adolesc Med. 2012;166:608–14. doi: 10.1001/archpediatrics.2011.1559. [DOI] [PubMed] [Google Scholar]
- 22.Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: The BABYDIET study. Diabetes Care. 2011;34:1301–5. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rewers M, Stene LC, Norris JM. Risk Factors for type 1 diabetes. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, et al., editors. Diabetes in America. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018. [PubMed] [Google Scholar]
- 24.Verhagen J, Smith EL, Whettlock EM, Macintyre B, Peakman M. Proinsulin-mediated induction of type 1 diabetes in HLA-DR4-transgenic mice. Sci Rep. 2018;8:14106. doi: 10.1038/s41598-018-32546-4. doi:10.1038/s41598-018-32546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonifacio E, Ziegler AG, Klingensmith G, Schober E, Bingley PJ, Rottenkolber M, et al. Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: The Pre-POINT randomized clinical trial. JAMA. 2015;313:1541–9. doi: 10.1001/jama.2015.2928. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler AG, Bonifacio E. BABYDIAB-BABYDIET Study Group. Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55:1937–43. doi: 10.1007/s00125-012-2472-x. [DOI] [PubMed] [Google Scholar]
- 28.Karl FM, Winkler C, Ziegler AG, Laxy M, Achenbach P. Costs of public health screening of children for presymptomatic type 1 diabetes in Bavaria, Germany. Diabetes Care. 2022;45:837–44. doi: 10.2337/dc21-1648. [DOI] [PubMed] [Google Scholar]
- 29.Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–91. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 30.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The diabetes prevention trial--Type 1. Diabetes Care. 2005;28:1068–76. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 31.Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: A randomized clinical trial. JAMA. 2017;318:1891–902. doi: 10.1001/jama.2017.17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: A double-blind, randomised controlled trial. Lancet. 2008;372:1746–55. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 33.Warshauer JT, Bluestone JA, Anderson MS. New frontiers in the treatment of type 1 diabetes. Cell Metab. 2020;31:46–61. doi: 10.1016/j.cmet.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381:603–13. doi: 10.1056/NEJMoa1902226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronson R, Gottlieb PA, Christiansen JS, Donner TW, Bosi E, Bode BW, et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: Results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37:2746–54. doi: 10.2337/dc13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:3285–96. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: A randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, et al. B-lymphocyte depletion with rituximab and β-cell function: Two-year results. Diabetes Care. 2014;37:453–9. doi: 10.2337/dc13-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, et al. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. 2020;383:2007–17. doi: 10.1056/NEJMoa2006136. [DOI] [PubMed] [Google Scholar]
- 40.A Study of SIMPONI®to Arrest Beta-cell Loss in Type 1 Diabetes-Full Text View-ClinicalTrials.gov. [[Last Accessed on 2023 Apr 16]]. Available from: https://clinicaltrials.gov/ct2/show/NCT02846545 .
- 41.Ke Q, Kroger CJ, Clark M, Tisch RM. Evolving antibody therapies for the treatment of type 1 diabetes. Front Immunol. 2020;11:624568. doi: 10.3389/fimmu.2020.624568. doi:10.3389/fimmu. 2020.624568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masharani UB, Becker J. Teplizumab therapy for type 1 diabetes. Expert Opin Biol Ther. 2010;10:459–65. doi: 10.1517/14712591003598843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cully M. T cell-regulating therapies for autoimmune diseases take FDA rejection in stride. Nat Rev Drug Discov. 2021;20:655–7. doi: 10.1038/d41573-021-00137-0. [DOI] [PubMed] [Google Scholar]
- 44.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 45.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: Metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–74. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, et al. Treatment of type 1 diabetes with teplizumab: Clinical and immunological follow-up after 7 years from diagnosis. Diabetologia. 2019;62:655–64. doi: 10.1007/s00125-018-4786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Office of the Commissioner. FDA Approves First Drug That Can Delay Onset of Type 1 Diabetes. U. S. Food and Drug Administration. [[Last accessed on 2023 Feb 20]]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-can-delay-onset-type-1-diabetes .
- 48.Teplizumab prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761183s000lbl.pdf .
- 49.Recent-Onset Type 1 Diabetes Trial Evaluating Efficacy and Safety of Teplizumab-Full Text View-ClinicalTrials.gov. [[Last accesed on 2023 Apr 16]]. Available from: https://clinicaltrials.gov/ct2/show/NCT03875729 .
- 50.Kühtreiber WM, Faustman DL. BCG therapy for type 1 diabetes: Restoration of balanced immunity and metabolism. Trends Endocrinol Metab. 2019;30:80–92. doi: 10.1016/j.tem.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Doupis J, Kolokathis K, Markopoulou E, Efthymiou V, Festas G, Papandreopoulou V, et al. The role of pediatric BCG vaccine in type 1 diabetes onset. Diabetes Ther. 2021;12:2971–6. doi: 10.1007/s13300-021-01163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang YC, Lin CJ, Hsiao YH, Chang YH, Liu SJ, Hsu HY. Therapeutic effects of BCG vaccination on type 1 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J Diabetes Res. 2020;2020:8954125. doi: 10.1155/2020/8954125. doi:10.1155/2020/8954125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elliott JF, Marlin KL, Couch RM. Effect of bacille Calmette-Guérin vaccination on C-peptide secretion in children newly diagnosed with IDDM. Diabetes Care. 1998;21:1691–3. doi: 10.2337/diacare.21.10.1691. [DOI] [PubMed] [Google Scholar]
- 54.Allen HF, Klingensmith GJ, Jensen P, Simoes E, Hayward A, Chase HP. Effect of bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care. 1999;22:1703–7. doi: 10.2337/diacare.22.10.1703. [DOI] [PubMed] [Google Scholar]
- 55.Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2:119–24. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- 56.D’Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: A multicenter analysis. Diabetes. 2014;63:3041–6. doi: 10.2337/db14-0295. [DOI] [PubMed] [Google Scholar]
- 57.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Jr, et al. Teplizumab for treatment of type 1 diabetes (Protégéstudy):1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katsumoto K, Yennek S, Chen C, Silva LFD, Traikov S, Sever D, et al. Wnt4 is heterogeneously activated in maturing β-cells to control calcium signaling, metabolism and function. Nat Commun. 2022;13:1–15. doi: 10.1038/s41467-022-33841-5. doi:10.1038/s41467-022-33841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singla R, Bindra J, Singla A, Gupta G, Gupta Y, Aggarwal S. Automation of insulin bolus dose calculation in type 1 diabetes: A feasibility study. Int J Diabetes Dev Ctries. 2022 doi:10.1007/s13410-022-01054-7. [Google Scholar]
- 60.Infante M, Baidal DA, Rickels MR, Fabbri A, Skyler JS, Alejandro R, et al. Dual-hormone artificial pancreas for management of type 1 diabetes: Recent progress and future directions. Artif Organs. 2021;45:968–86. doi: 10.1111/aor.14023. doi:10.1111/aor. 14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mital S, Nguyen HV. Cost effectiveness of teplizumab for prevention of type 1 diabetes among different target patient groups. Pharmacoeconomics. 2020;38:1359–72. doi: 10.1007/s40273-020-00962-y. [DOI] [PubMed] [Google Scholar]